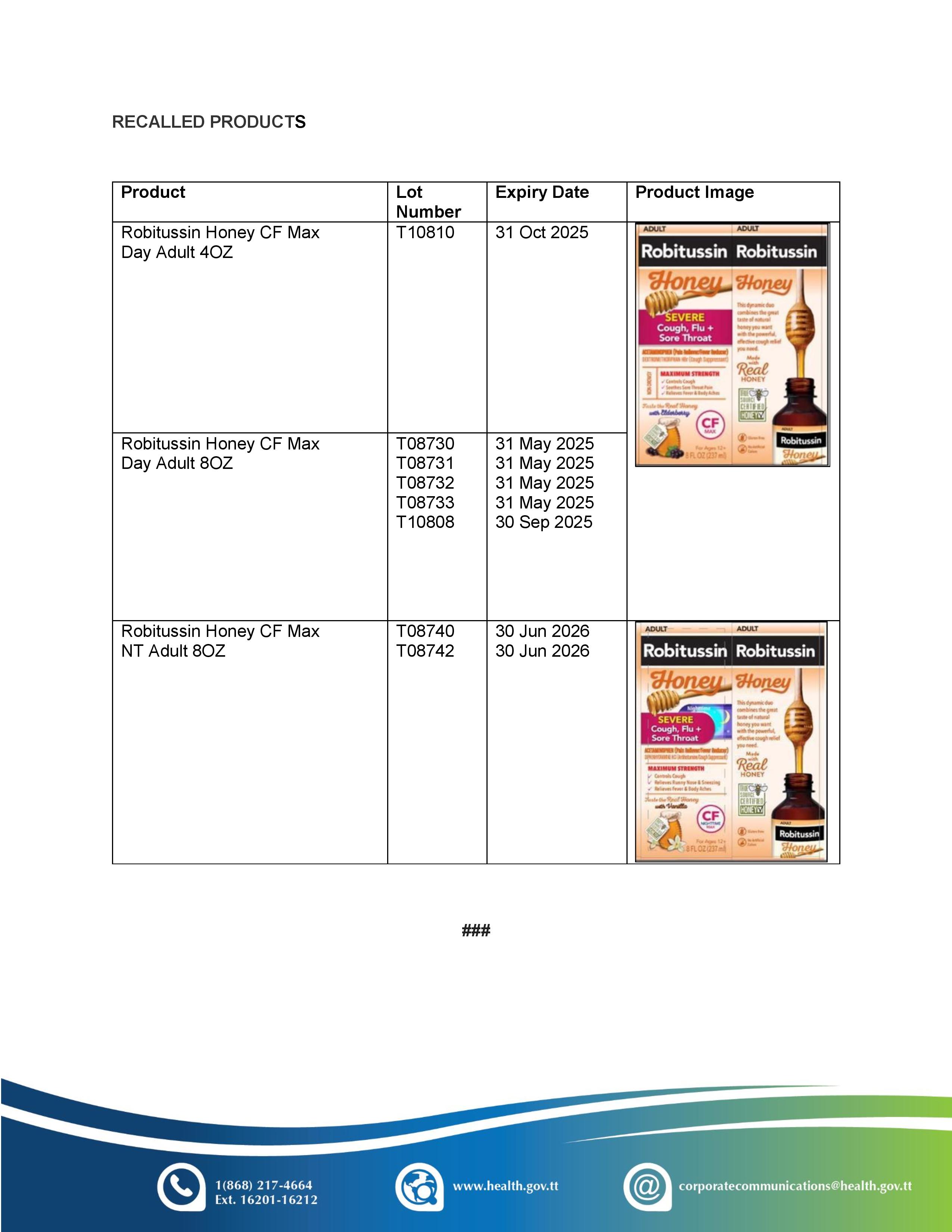
Port of Spain, February 20, 2024: The Ministry of Health (MoH), via its Chemistry, Food and Drugs Division, advises of a voluntary recall notice for eight lots of Robitussin Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult. The recall was issued by the manufacturer Haleon, due to microbial contamination.
Robitussin is used for cough relief. For immunocompromised individuals, the use of these contaminated products may result in adverse effects such as fungemia or disseminated fungal infection. Although life-threatening infections are unlikely to occur in non-immunocompromised persons, the occurrence of an infection that may require medical intervention cannot be completely ruled out.
Robitussin Honey CF Max Day Adult, (4 and 8 ounce) is registered for sale in this country. Out of an abundance of caution, the Ministry is urging persons who may be in possession of these recalled products to discontinue use immediately and to return the product to the place of purchase where possible.
Additional Information can be obtained by contacting the Office of the Director of the Chemistry, Food and Drugs Division via email at cfdd@health.gov.tt or phone at 217-4664 ext. 13101.
The Ministry of Health will continue to monitor the situation and advise the population as necessary.