Port of Spain, October 24, 2024: The Ministry of Health (MoH), via its Chemistry, Food and Drugs Division advises of the voluntary recall of 26 lots of "Apo Amitriptyline 10mg and 25mg Tablets.
The recall issued by Apotex Incorporated, the manufacturer of the product, is as a result of exceeded limits of N-Nitroso Nortriptyline (NNORT), which was found in three (3) lots of the drug Apo-Amitriptyline. The additional 23 lots were recalled by the manufacturer as a measure of caution.
The Ministry of Health via its Chemistry, Food and Drugs Division, has been in contact with the local distributor Ultra-Pharm Marketing Limited, who have advised that all retail establishments have been contacted and instructed to suspend all sales of the drug immediately. The distributor has also advised retail establishments to remove the drug from point of sale and return to the distributor by Friday October 25, 2024.
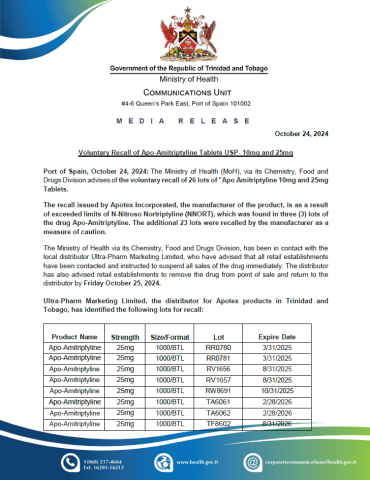
Ultra-Pharm Marketing Limited, the distributor for Apotex products in Trinidad and Tobago, has identified the following lots for recall:
Product Name | Strength | Size/Format | Lot | Expire Date |
| Apo-Amitriptyline | 25mg | 1000/BTL | RR0780 | 3/31/2025 |
| Apo-Amitriptyline | 25mg | 1000/BTL | RR0781 | 3/31/2025 |
| Apo-Amitriptyline | 25mg | 1000/BTL | RV1656 | 8/31/2025 |
| Apo-Amitriptyline | 25mg | 1000/BTL | RV1657 | 8/31/2025 |
| Apo-Amitriptyline | 25mg | 1000/BTL | RW8691 | 10/31/2025 |
| Apo-Amitriptyline | 25mg | 1000/BTL | TA6061 | 2/28/2026 |
| Apo-Amitriptyline | 25mg | 1000/BTL | TA6062 | 2/28/2026 |
| Apo-Amitriptyline | 25mg | 1000/BTL | TF8602 | 8/31/2026 |
| Apo-Amitriptyline | 25mg | 1000/BTL | TF9603 | 8/31/2026 |
| Apo-Amitriptyline | 10mg | 1000/BTL | RR0265 | 3/31/2025 |
| Apo-Amitriptyline | 10mg | 1000/BTL | RVl644 | 8/31/2025 |
| Apo-Amitriptyline | 10mg | 1000/BTL | RW8597 | 11/30/2025 |
| Apo-Amitriptyline | 10mg | 1000/BTL | RW8598 | 11/30/2025 |
| Apo-Amitriptyline | 10mg | 1000/BTL | TA6009 | 1/31/2026 |
| Apo-Amitriptyline | 10mg | 1000/BTL | TF8586 | 7/31/2026 |
| Apo-Amitriptyline | 10mg | 1000/BTL | TF8588 | 7/31/2026 |
| Apo-Amitriptyline | 10mg | 1000/BTL | TF8589 | 7/31/2026 |
Out of an abundance of caution, the Ministry advises persons who may be in possession of the drug ApoAmitriptyline with the identified lot numbers to discontinue use immediately and to return the product to the place of purchase where possible.
Additional information can be obtained by contacting the Office of the Director of the Chemistry, Food and Drugs Division via email at cfdd@health.gov.tt or phone at 217-4664 ext. 13101.
The Ministry of Health will continue to monitor the situation and advise the population as necessary.
###