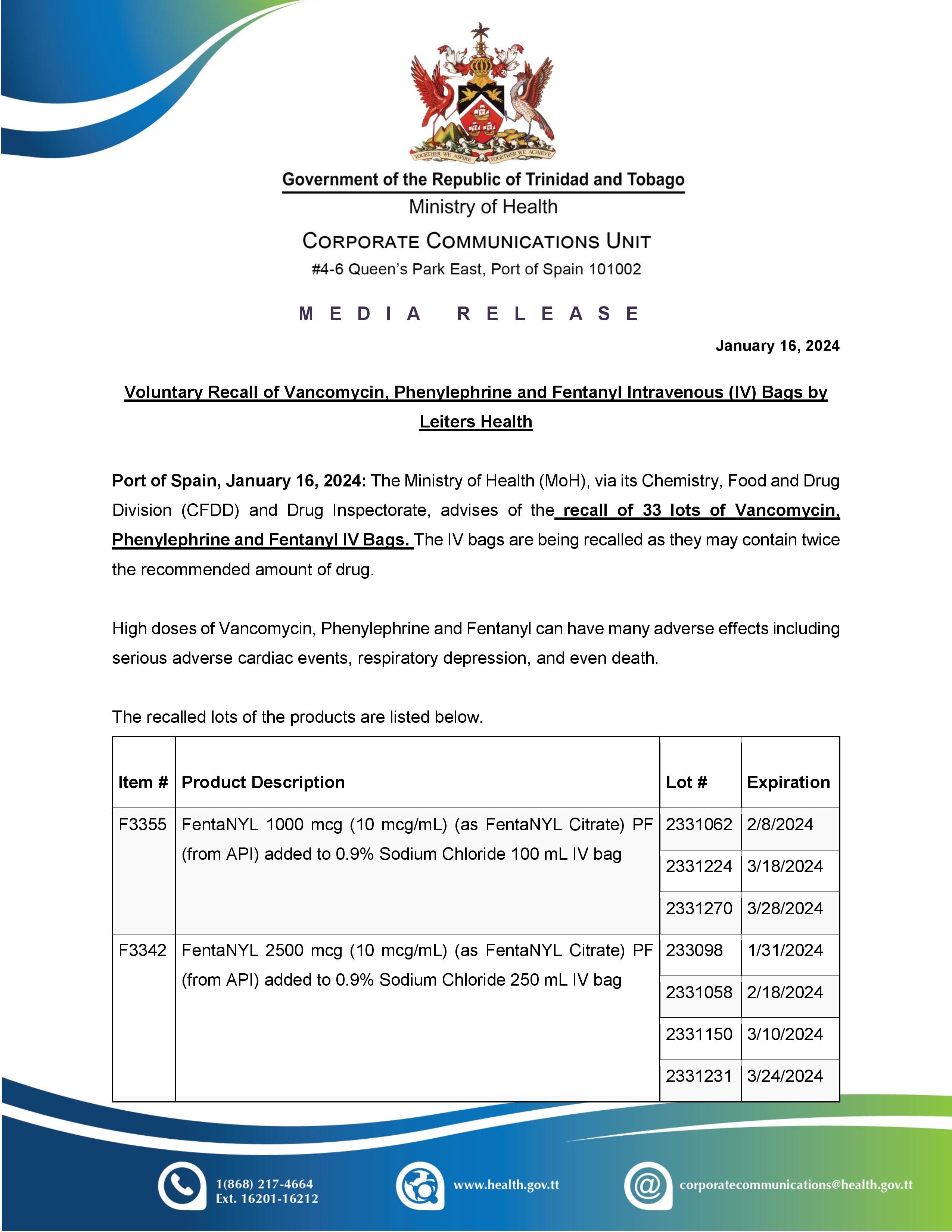
Media Release - Voluntary Recall Notice of Zenzedi® (dextroamphetamine sulfate tablets, USP)
Port of Spain, February 9, 2024: The Ministry of Health (MoH), via its Chemistry, Food and Drugs Division, advises of a voluntary recall notice of Zenzedi CII (dextroamphetamine sulfate tablets, USP) 30 mg. The recall was issued by Azurity Pharmaceuticals, Inc. due to the drug Carbinoxamine Maleate, which was found in the packaging.
Zenzedi is a prescription medicine for the treatment of Narcolepsy (sleep disorder) as well as for Attention Deficit Hyperactivity Disorder (ADHD).